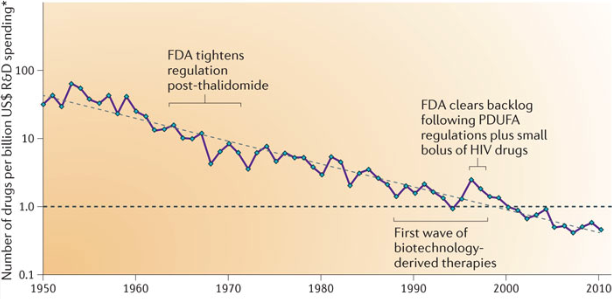
There is plenty of blame to go around for the Covid pandemic, but one factor that has so far escaped notice is Eroom’s Law (Eroom is “Moore” spelled backwards). Since the 1950s, the number of drugs approved per dollar spent in research and development has been declining exponentially (inflation-adjusted, of course).
Eroom’s Law means that we are missing out on valuable life-saving and quality-of-life enhancing treatments. Many of these potential treatments could also save money, as quickly curing a disease is often less expensive than prolonged care for a chronic condition. It also means, as we will see below, that the state of the art in the medical clinic greatly lags what is possible in our nation’s biology laboratories.
One of the main explanations for Eroom’s Law is what the authors of the paper that defined it call the “cautious regulator” problem. Over time, the Food and Drug Administration has progressively lowered its risk tolerance, making new drug development costlier and harder. To be sure, the FDA has been blamed for its failures in the Covid pandemic—but mainly for more immediate errors, like restricting lab testing, not allowing challenge trials, and being slow to approve vaccines and therapeutics. The cumulative error of becoming continuously more cautious is perhaps the most serious one.
Once the genome of the novel coronavirus was sequenced, it took only two days for Moderna to design its mRNA vaccine. When the vaccines were finally approved months later, David Wallace-Wells reports, “None of the scientists I spoke to for this story were at all surprised by either outcome—all said they expected the vaccines were safe and effective all along.”
Even though the vaccine design was ready in January 2020, the mRNA supply chain wasn’t. Thanks to Eroom’s Law, the Covid vaccines were the first mRNA treatments approved for humans, so the factories, equipment, and ingredients necessary for producing billions of doses were not yet available.
It could have been otherwise. mRNA transfection using a lipid nanoparticle was first demonstrated in 1989. It was used to trigger an immune response in mice in 1993, and against a virus in 1994. The first human trials, against prostate cancer, happened in 2001. Yet it took a global pandemic to get mRNA out of the lab and into the clinic.
Imagine a counterfactual in which Eroom’s Law did not hold. Suppose that drug commercialization was orders of magnitude more efficient, as it was in the 1950s and 1960s. It’s easy to imagine that the first human mRNA treatments could have been approved in the 1990s. They could have been perfected in the 2000s, and they would have been utterly commonplace in the 2010s.
By the time the Covid virus showed up in late 2019, the supply chain would already have been built out. We would have had mRNA vaccine platforms that were already well tested. We could have quickly vaccinated key populations and perhaps even suppressed the spread of the virus, preventing it from becoming endemic at a comparatively low economic (and human) cost. If we failed to suppress the spread, we could at least always have had vaccines that matched the variants that were currently in circulation, reducing the need for other mitigation methods.
Extrapolating to the future
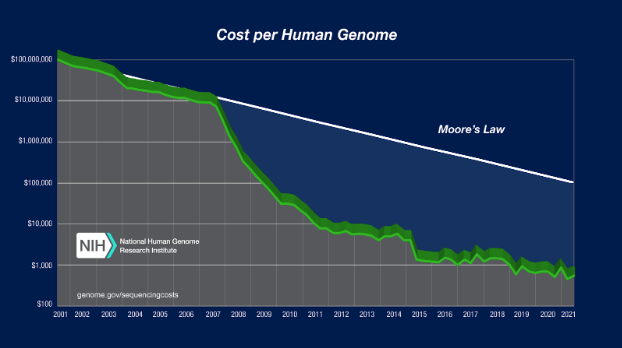
The exponential slowdown in drug R&D effectiveness stands in stark contrast to what is going on in our nation’s biology labs. Progress in molecular genetics has been superexponential. In 2001, the cost to sequence a human genome was around $100 million. In 2022, it is around $500. Today’s scientists can not only sequence genomes, they can use tools like CRISPR to modify the DNA of living organisms.
Outside of DNA sequencing, there has been similar rapid progress. In the laboratory, scientists have developed synthetic, programmable lifeforms. They can make organisms regrow a severed limb—or grow a second head instead. They have developed artificial wombs that can sustain lamb fetuses. They can reanimate pig brains and other organs after the pig’s heart has stopped for an hour. They can make adult cells revert back to undifferentiated stem cells, and they are starting to be able to make them revert only part of the way, back to a youthful but still differentiated state. There are countless more advances being made every day in the lab.
If Eroom’s Law were not a factor, many of these developments would soon make their way into medical practice. Instead, personal genomics companies like 23andMe need FDA approval to tell you only a tiny sliver of what we know about what your genes mean for your health risks. There are still no approved CRISPR therapies in humans. A recent New York Times article on pig reanimation spent half its column-inches interviewing bioethicists who explained, not to worry, that it would be a long time before this technology would be used in humans—potentially to save soldiers who bleed out on the battlefield or child drowning victims.
The gap between what is possible in the biology lab and what is available in the medical clinic keeps growing wider as a result of these diverging exponential trends.
Because of this gap, biological terrorism is now conceivable on a much greater scale than it was a few decades ago. Rapid progress in the lab means that it is now within scientists’ power to resurrect the smallpox virus or to design an even deadlier one. That’s scary, but the effectiveness of such terrorism depends on our ineffectiveness in providing treatments.
So far, “biosecurity” discussions have placed considerable attention on preventing or controlling certain research, such as gain-of-function, that could result in a future pandemic. However, at least an equally important lever in fighting future pandemics comes from rapidly expanding the set of medical treatments that are available in the medical clinic. If Eroom’s Law were reversed, fast progress in our biology labs could be just as quickly translated into treatments that improve human lives now and make us more secure in a future biosecurity event.
It’s a golden age for biology. Our scientists can do truly amazing things. It would be a shame if, when a more serious pandemic strikes, the tools we need to fight it are stuck in a lab somewhere.